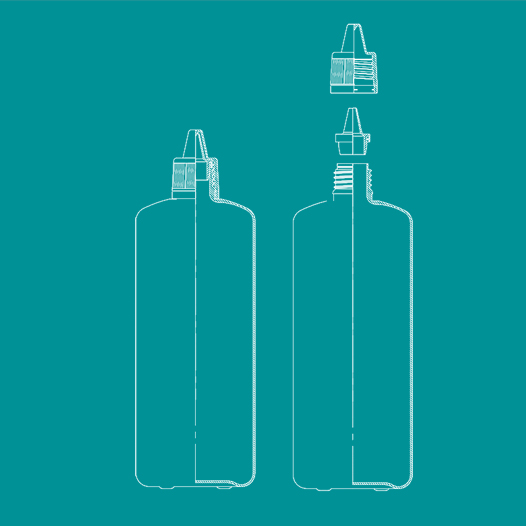
Plastic bottles (plastic containers) with tamper-evident band for pharmaceutical use are used for packing (storing) liquid pharmaceutical products (magistral medicine, galenical medicine, ready-made medicines, and pharmaceutical substance). They can be in direct contact with the product. These containers are „tamper-proof“ (closures with tamper-evident band) because they ensure that the container is original and not opened. The pharmaceutical plastic containers are made from materials that are described in Ph.Eur. and they meet the criteria of the specific use assessment that are also described in Ph.Eur. We buy the materials from highly reliable producers. Bottles under catalogue numbers 21010, 21020, 21030 are made from low-density polyethylene and they meet the pharmaceocopeic requirements. They are used for storing multi-dose packets of ophthalmological products (10-30ml), i.e. non-sterile liquid pharmaceutical products. The conditions and the method of production of plastic containers and closures are in accordance with the following standards: ISO 9001, ISO 14001, and OHSAS 18001. Customers can choose the colour of the container (proof of additives) and whether they want containers to be sterile or not. With the containers we deliver producer´s certificate that is accompanied by technical documentation and documentation which states that the final products and materials they are made from are safe for health (traceability of product´s documentation).